AS-Level Chemistry Chapter 4 Chemical Bonding Intermolecular Forces Hydrogen Bonding
To view other notes related to AS-Level Chemistry. Please Click Here.
Intermolecular Forces
Intermolecular and intramolecular forces
2) Intermolecular force is the force of attraction between one molecule and the neighboring molecule.
3) There are several types of intermolecular forces:
4) Intermolecular forces are much weaker than intramolecular forces.
5) Intermolecular forces are responsible for the melting and boiling points of substances, as well as their physical states.
Permanent dipole-dipole forces(Keesom forces)
1) Polar molecules have a negative end and a positive end. The oppositely-charged ends will attract one another. The forces of attraction is called permanent dipole-dipole forces.
2) Only polar molecules will experience permanent dipole-dipole forces.
Temporary dipole-induced dipole forces(Dispersion forces or London forces)
1) Electrons are mobile, and constantly revolving around the nucleus. Even in nonpolar molecules, there is a high possibility that at any given instant, the electron density is higher on one side than the other.
2) This will result in the formation of temporary dipole(or instantaneous dipole) because it only lasts for just a tiny fraction of time.
3) In the next instance, the distribution of electron density will change and the molecule has a new temporary dipole.
4) The temporary dipole set up can distort the electron charge clouds of the neighboring molecules, giving rise to induced dipoles.
5) The forces of attraction between temporary dipoles and induced dipoles give rise to dispersion forces.
6) All molecules(polar and non-polar) will experience dispersion forces. For polar molecules, permanent dipole-dipole forces exist in addition with dispersion forces.
Factors affecting strength of dispersion forces
1) Number of electrons in the molecule(Size of molecule)
- This causes the attraction between the nucleus and the electrons to get weaker, the electrons become progressively easier to be distorted.
- This causes more temporary dipoles to be set up and the dispersion forces get stronger.
- The boiling point of halogens increases going down the Group. The size of the molecules increases and thus the van der Waals' forces become stronger.
2) Number of contact points between the molecules(Surface area)
Hydrogen bonding
1) Hydrogen bond is the electrostatic force of attraction between a hydrogen atom (which is covalently bonded to a small and highly electronegative atom) and the lone pair of electrons of another small and highly electronegative atom.
2) The 'small and highly electronegative atoms' are fluorine, oxygen and nitrogen.
3) The conditions necessary for forming hydrogen bonds:
ii. a lone pair of electrons from the small and electronegative atom.
4) The attraction between the hydrogen atom and the lone pair of electrons constitutes the hydrogen bond.
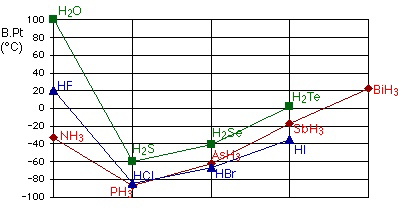
5) The evidence of hydrogen bonding: - H2O, HF and NH3 have exceptionally high boiling points compared to other compounds with greater number of electrons.
- This additional intermolecular force is called the hydrogen bond.
6) H2O has a higher boiling point compared to NH3 and HF. This is because a H2O molecule can form, on average four hydrogen bonds. In NH3, the number of hydrogen bonds is restricted by the one lone pair of electrons in the NH3 molecule. In HF, it is restricted by the number of hydrogen atoms.